Zolgensma: A Breakthrough Gene Therapy for SMA
October 4, 2023
CAR T Cell Therapy: A Breakthrough in Cancer Immunotherapy
October 4, 2023What is Luxturna?
Luxturna is a prescription gene therapy product that is used to treat vision loss due to inherited retinal disease caused by mutations in both copies of the RPE65 gene1. The RPE65 gene is responsible for producing a protein that helps the retina (the light-sensitive layer at the back of the eye) convert light into electrical signals that are sent to the brain. When the RPE65 gene is mutated, the retina cannot function properly and vision deteriorates over time2.
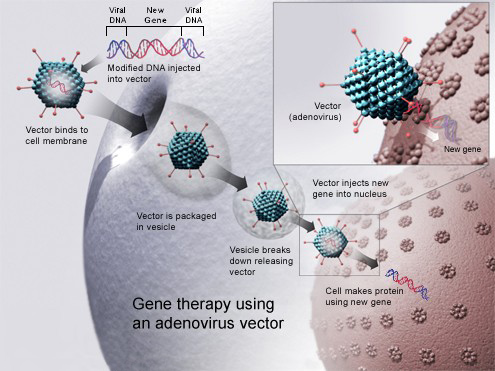
Luxturna works by delivering a normal copy of the RPE65 gene to the retinal cells using a modified virus called adeno-associated virus serotype 2 (AAV2)2. The virus acts as a vector, or carrier, that transfers the gene into the cells. Once inside, the gene instructs the cells to produce the missing protein and restore some of the retina’s function1.
Luxturna is not a cure for inherited retinal disease, but it can improve vision and quality of life for people who have enough remaining retinal cells1. Luxturna is administered as a one-time subretinal injection in each eye, performed by a surgeon in a sterile setting1.
Who can benefit from Luxturna?
Luxturna is indicated for adults and children with vision loss due to inherited retinal dystrophy caused by confirmed biallelic RPE65 mutations and who have sufficient viable retinal cells3. Biallelic means that both copies of the RPE65 gene are mutated, one inherited from each parent. This condition is also known as Leber congenital amaurosis type 2 or retinitis pigmentosa type 202.
Inherited retinal disease due to RPE65 mutations is a rare genetic disorder that affects about 1,000 to 2,000 people in the United States and about 3,000 people in Europe4. It usually manifests in early childhood and leads to progressive vision loss, night blindness, tunnel vision, and eventually blindness2.
To be eligible for Luxturna, patients must undergo genetic testing to confirm that they have biallelic RPE65 mutations and an eye examination to determine that they have enough viable retinal cells1. Genetic testing can also help identify family members who may be carriers of the mutation or who may be at risk of developing the condition4.
What are the results of Luxturna?
Luxturna was approved by the US Food and Drug Administration (FDA) in December 2017, making it the first in vivo gene therapy approved by the FDA. It was also approved by the European Medicines Agency (EMA) in November 2018 and by Health Canada in October 20203 .
The approval of Luxturna was based on the results of a phase III clinical trial that involved 31 patients with inherited retinal disease due to RPE65 mutations. The trial compared the efficacy and safety of Luxturna with a control group that received a sham injection (an injection without any active substance).
The primary outcome measure was the change in functional vision as assessed by a multi-luminance mobility test (MLMT), which evaluates the ability to navigate an obstacle course under various levels of illumination. The secondary outcome measures included visual acuity, light sensitivity, visual field, and quality of life.
The trial showed that Luxturna significantly improved functional vision compared with the control group at one year after treatment. The improvement was maintained at three years after treatment. Luxturna also improved visual acuity, light sensitivity, visual field, and quality of life in most patients .
What are the side effects of Luxturna?
The most common side effects of Luxturna are eye redness, cataract, increased intraocular pressure, and retinal tear1. These side effects are usually mild to moderate and can be managed by eye drops or surgery if needed1.
Some serious side effects that may occur during or after the administration of Luxturna include eye infection, permanent decline in visual acuity, changes or damage to the retina, detachment of the retina, or expansion of the air bubble formed in the eye after injection. These side effects may require urgent medical attention and may lead to blindness if not treated promptly.
Patients who receive Luxturna should follow up with their healthcare professional as instructed to monitor their eye health and report any signs or symptoms of complications1. Patients should also avoid air travel, travel to high elevations, or scuba diving until the air bubble in the eye has disappeared1.
How to access Luxturna?
Luxturna is a highly specialized and expensive treatment that is only available at selected centers that have the expertise and facilities to administer it safely and effectively. Luxturna costs $425,000 per eye in the United States. The price may vary in other countries depending on the reimbursement policies and negotiations with the manufacturer, Spark Therapeutics4.
Patients who are interested in Luxturna should consult with their healthcare professional to determine if they are eligible for the treatment and to find out how to access it in their region4. Patients can also contact Spark Therapeutics Generation Patient Services℠, a support program that provides information, education, and assistance for eligible patients who receive Luxturna.
Conclusion
Luxturna is a breakthrough gene therapy product that can improve vision and quality of life for people with inherited retinal disease due to RPE65 mutations. Luxturna is not a cure, but it can restore some of the retina’s function and slow down the progression of vision loss. Luxturna is a one-time treatment that is administered as a subretinal injection in each eye. Luxturna has been shown to be safe and effective in clinical trials, but it may cause some side effects that require medical attention. Luxturna is a rare and costly treatment that is only available at specialized centers. Patients who are interested in Luxturna should consult with their healthcare professional and contact Spark Therapeutics Generation Patient Services℠ for more information.