Medical Tourism: What Is It and Why Is It Beneficial?
October 4, 2023
How Luxturna Can Brighten Up the Night for People with Inherited Retinal Disease
October 4, 2023Spinal muscular atrophy (SMA) is a rare and devastating genetic disease that affects the motor neurons responsible for muscle functions such as breathing, swallowing, speaking and walking. It is caused by a mutation or deletion in the survival motor neuron 1 (SMN1) gene, which leads to a deficiency of the survival motor neuron (SMN) protein. Without enough SMN protein, the motor neurons die, resulting in progressive muscle weakness and paralysis. SMA is the leading genetic cause of infant mortality, and most children with the most severe form of SMA do not survive past early childhood.
What is Zolgensma?
Zolgensma (onasemnogene abeparvovec-xioi) is a gene therapy that was approved by the FDA in 2019 and by Health Canada in 2020 for the treatment of pediatric patients with SMA. It is indicated for children less than two years of age with bi-allelic mutations in the SMN1 gene and three or fewer copies of the SMN2 gene, or infantile-onset SMA. Zolgensma is designed to address the genetic root cause of SMA by delivering a new, working copy of the human SMN1 gene into the target motor neuron cells. This restores the production of SMN protein and halts the disease progression.
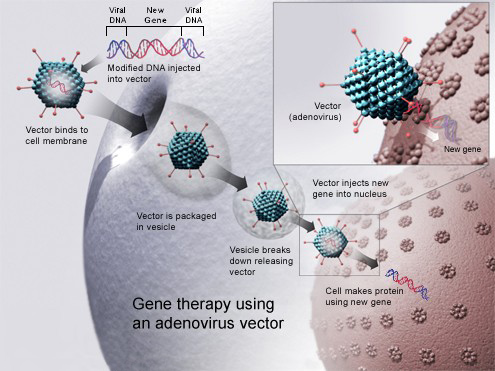
Zolgensma is administered as a one-time intravenous infusion that takes about an hour. It is a vector-based gene therapy that uses an adeno-associated virus (AAV) to carry the SMN1 gene into the cells. The AAV vector is modified to remove its ability to cause disease or replicate, and it only delivers the gene to the cells without integrating into the DNA.
How effective is Zolgensma?
Zolgensma has shown remarkable results in clinical trials and real-world evidence. In the pivotal phase 3 STR1VE trial, Zolgensma demonstrated significant improvement in survival and motor milestones in patients with SMA type 1. At 14 months of age, 100% of patients who received Zolgensma were alive without permanent ventilation, compared to only 8% of untreated patients in a historical cohort. Moreover, 91% of patients who received Zolgensma achieved at least one motor milestone, such as sitting without support, rolling over or crawling, compared to 0% of untreated patients.
In addition to STR1VE, Zolgensma has also shown efficacy in other clinical trials, such as START and SPR1NT. In START, a phase 1 trial that enrolled patients with SMA type 1 who were younger than six months at the time of treatment, Zolgensma resulted in prolonged survival and improved motor function. At five years of age, all patients who received Zolgensma were alive and free of permanent ventilation, and most of them could sit independently and communicate verbally. In SPR1NT, a phase 3 trial that enrolled presymptomatic patients with SMA who had two or three copies of SMN2 gene, Zolgensma led to rapid and sustained achievement of motor milestones that were consistent with normal development. All patients who received Zolgensma were alive and free of permanent ventilation at 18 months of age, and most of them could sit, stand and walk independently.
Zolgensma has also demonstrated its effectiveness in real-world settings, where it has been used to treat more than 600 patients with SMA across different countries. According to data from Novartis, the manufacturer of Zolgensma, patients who received Zolgensma have shown sustained improvement in survival and motor function, as well as reduced hospitalizations and respiratory interventions.
What are the side effects of Zolgensma?
Zolgensma is generally well tolerated, but it may cause some side effects that require monitoring and management. The most common side effects are elevated liver enzymes and vomiting. Elevated liver enzymes indicate that the liver is inflamed or damaged by the AAV vector or the immune response to it. This can be detected by regular blood tests and treated with steroids. Vomiting may occur due to the infusion or the steroids, and it can be managed with anti-nausea medications.
Other possible side effects include low platelet count, fever, rash, joint pain and allergic reactions. These are usually mild to moderate and resolve within a few days or weeks. However, some rare but serious side effects may occur, such as bleeding, infection or anaphylaxis. Therefore, patients who receive Zolgensma should be closely monitored by their health care providers before, during and after the infusion.
What is the cost of Zolgensma?
Zolgensma is one of the most expensive drugs in the world, with a list price of $2.1 million USD per patient. However, this price reflects the value of a one-time treatment that can potentially cure a life-threatening disease and save the costs of long-term care and interventions. Novartis has also offered various options to make Zolgensma more accessible and affordable, such as outcome-based agreements, installment payments and patient assistance programs.
In Canada, Zolgensma is not yet covered by public or private insurance plans, but Novartis is working with the health authorities and stakeholders to secure reimbursement and funding. In the meantime, Novartis has launched a managed access program (MAP) to provide Zolgensma to eligible patients with SMA type 1 who are under six months of age and meet the criteria for treatment. The MAP is a compassionate use program that allows patients to access Zolgensma for free until it is approved for funding by the health system.
Conclusion
Zolgensma is a breakthrough gene therapy that offers a new hope for children with SMA. It is a one-time treatment that can stop the disease progression and improve the survival and quality of life of patients. Zolgensma has shown remarkable results in clinical trials and real-world evidence, and it has been approved by several regulatory agencies around the world. However, Zolgensma is also very expensive and not widely available, and it may cause some side effects that require monitoring and management. Therefore, patients who are interested in Zolgensma should consult their health care providers and discuss the benefits and risks of this therapy.